Global public health improved thanks to anti-tuberculosis drug, delamanid
Tuberculosis (TB) has been the scourge of humankind for millenia. There’s evidence that it existed in ancient Egypt.*1 It is the world’s second leading infectious killer after COVID-19.*2,3 Drug-resistant strains proliferating in recent decades have rendered TB even more lethal.
TB is, however, curable and preventable. By deploying its anti-tuberculosis drug, delamanid, the Otsuka group is seeking to go even further and eliminate TB.
- *1Thomas M. Daniel (2006)
- *2World Health Organization. Global tuberculosis report 2020
- *3World Health Organization. Coronavirus disease (COVID-19) dashboard
What is tuberculosis?
TB is one of the most serious infectious diseases in the world, where Mycobacterium tuberculosis (M.tb) enters the body and multiplies, mainly in the lungs, resulting in symptoms such as coughing and production of sputum. In serious cases, the infection can cause breathing difficulties and affect other organs, potentially resulting in death. While highly contagious, about 5-10% of infected people will develop the disease at some time in their lives. Some of them may develop the disease several years after infection due to declining physical strength or aging.
Multidrug-Resistant and Extensively Drug-Resistant TB
Since the 1990s, M.tb bacteria have emerged which have acquired widespread resistance to anti-tuberculosis drugs. Discontinuation of medication for any reason, such as irregular doses or tolerability, gives rise to bacteria that are resistant to the drugs that have been taken. Among these are multidrug-resistant TB (MDR-TB) bacteria that have become resistant to rifampicin and isoniazid, the two most potent first-line therapeutic agents. Extensively drug-resistant TB (XDR-TB) is resistant to an even greater number of drugs, representing a major global challenge to efforts to control the disease.
SDGs target 3.3
In 2015, the United Nations set out its Sustainable Development Goals. SDG 3 addressed public health. SDG 3.3 set the year 2030 as the target to end the epidemics of AIDS, TB, malaria and neglected tropical diseases, as well as to combat hepatitis, waterborne infections and other communicable diseases. However, modeling from the Stop TB Partnership on COVID-19 disruptions to TB services in countries with a high burden of the disease, indicates that the 2030 target may be set back at least five years.*4
- *4"TB and COVID-19" Stop TB Partnership website, available at: http://www.stoptb.org/covid19.asp
Number of individuals infected with Mycobacterium tuberculosis: approx.1.7billion
Annual deaths from tuberculosis: approx. 1.5 million
TB still spreading worldwide
TB is highly contagious. About 1.7 billion people are said to be infected with M.tb bacteria in the world. An estimated 9.9 million people develop TB in 2020, of whom about 1.5 million die.*5
TB is prevalent in many low- and middle-income countries. Because TB requires long-term treatment, it represents a major economic challenge in high burden countries.
- *5World Health Organization. Global Tuberculosis Report 2021
Limitations of conventional medicinal treatment
M.tb, which may develop into active TB disease, is a very “stubborn bacterium.” Currently, treatment usually involves administering three to four drugs, to suppress the risk of the patient developing resistance to any one of them. In most cases, drug-sensitive TB can be successfully treated with what are known as “first-line drugs” and proper case management. However, for the more dangerous MDR- and XDR-TB strains, doctors turn to “second-line” drugs, and those combinations can include delamanid.
Delamanid is improving global health
One of the first new drugs in 40 years
Birth of delamanid
“If nobody does it, Otsuka must do it.”
Delamanid first received approval in 2014 in the European Union for the treatment of adult, pulmonary MDR-TB. It has a completely different mechanism of action compared with previous therapeutic agents, and is also effective against TB bacteria that have become resistant to existing drugs. Since 2015, delamanid has been included in the WHO Model List of Essential Medicines (list of priority drugs in any country).
Delamanid is one of the newest anti-tuberculosis drugs approved in the world in the past 40 years. Although MDR-TB is currently gaining prominent attention, it had been thought that TB had become a disease that humankind has already overcome since rifampicin, currently used as one of the first-line drugs, was discovered in 1964. In the 1970s, when many researchers and research institutes around the world stopped development, Otsuka Pharmaceutical continued research, based on the belief that “TB is a serious global health problem, and we must continue our research if nobody else does it.” Delamanid was created after more than 30 years. Otsuka Pharmaceutical remains actively engaged in Research & Development on new anti-tuberculosis drugs. For more than a decade, the company has consistently ranked among the top funders of TB R&D worldwide.
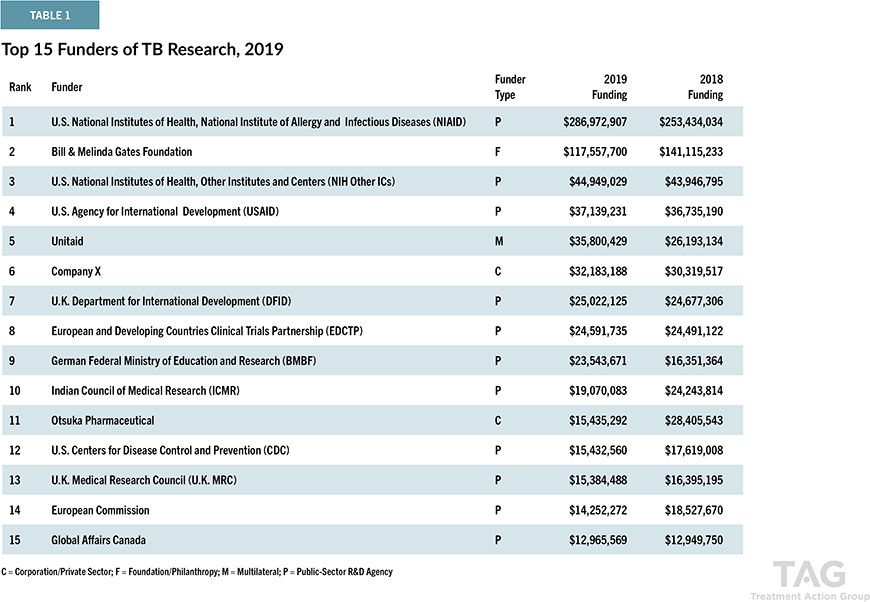
Tuberculosis Research Funding Trends 2005 – 2019, Stop TB Partnership and Treatment Action Group
A brief history of Otsuka Pharmaceutical’s Global TB Programme
| 1971 | TB selected as one of the company’s earliest research priorities |
|---|---|
| 2002 | Otsuka Pharmaceutical researchers find a new anti-TB drug candidate compound |
| 2004 | Phase I clinical trial of OPC-67683, (later named delamanid) starts |
| 2008 | Phase II clinical trial for MDR-TB patients starts |
| 2011 | Phase III clinical trial for MDR-TB patients starts |
| 2013 | Clinical trials for paediatric patients with MDR-TB start |
| 2014 | Approval granted in the European Union and Japan for treatment of pulmonary MDR-TB in adult patients |
| 2016 | Phase I of OPC-167832, a second new anti-TB compound starts |
| 2018 | Phase I/II of OPC-167832 starts |
| 2020 | Participate in the world's first-of-its kind collaboration, the PAN-TB Consortium, that transcends industry boundaries |
Delamanid for children
Between 25,000 and 32,000 children develop MDR-TB each year.*6 Of these, only 3 - 4 per cent are diagnosed and treated and consequently approximately 21 per cent of children with MDR-TB are likely to die.*7
The treatment for MDR-TB is arduous and particularly so for children.
Otsuka Pharmaceutical has developed a novel paediatric formulation of delamanid. In 2021, it was approved by the European Medicines’ Agency (EMA) for use in children weighing at least 10 kgs.
- *6Access to Medicine Foundation report "Tuberculosis in Children: Underdiagnosed and Undertreated" (2020)
- *7Helen E Jenkins and Courtney M Yuen (2018)
External Collaborations
Expanding access to tuberculosis drugs in low-income countries in collaboration with the Stop TB Partnership’s Global Drug Facility *8

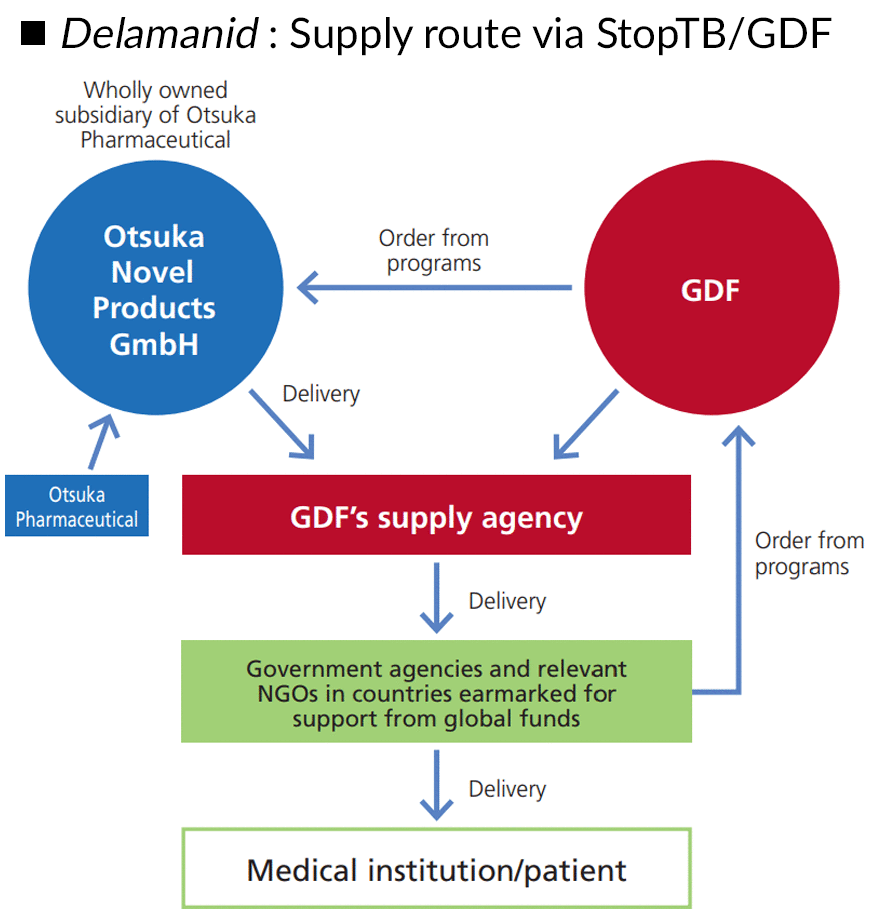
Delamanid is one of the first new anti-tuberculosis drugs approved in the past 40 years, but this is meaningless if the drug is not accessible to patients. That said, there are many TB patients in developing countries of Africa, Asia, and other regions where the Otsuka Group does not have a business base. For this reason, we work with the Stop TB Partnership’s Global Drug Facility (GDF), an organization dedicated to expanding access to quality-assured anti-tuberculosis drugs and diagnostic agents, and ensuring the sustainable procurement of those drugs in developing countries. GDF now supplies delamanid to more than 80 countries, and the majority of patients currently taking this drug receive their medicines through GDF, highlighting the organization’s excellent contribution to expanding access.
- *8Founded in 2001, the Stop TB Partnership has a mission to serve every person who is vulnerable to TB and to ensure that high-quality diagnosis, treatment and care are available to all who need it. GDF is the largest global provider of quality-assured anti-tuberculosis medicines, diagnostics, and laboratory supplies to the public sector while also providing technical assistance to TB programs and supporting wide use of innovative tools.
■DELTYBA : Supply route via StopTB/GDF
Cooperation with Japanese government on delamanid

We also cooperate with, and receive support from, the Japanese government in various ways. These include support for access initiatives overseas and sharing educational information at international conferences and events.

One of the eight points in the economic cooperation plan agreed by Japanese and Russian leaders is to improve medical care and promote healthy life spans. At the Eastern Economic Forum in Vladivostok on September 7, 2017, Japan’s Prime Minister, Shinzo Abe, spoke of mutual cooperation, aimed at getting swift approval for delamanid in Russia. In that speech, he said, “In the fight against TB, Japan and Russia have joined hands.” Delamanid received regulatory approval in the Russian Federation in 2020.
At the United Nations General Assembly High-Level Meeting on Tuberculosis in New York City on September 26, 2018, Katsunobu Kato, Minister of Health, Labour and Welfare, gave a speech on developing treatments for MDR-TB in Japan and contributing to eradication of the disease worldwide, and at the same time adopted the political declaration toward eliminating TB.
Establishing a supply network which will save patients around the world
Expanding access to patients worldwide

Numerous collaborations have been formed to combat TB and other diseases that threaten global health, for example with the WHO, United Nations and national governments who provide active support. In addition to its cooperation with GDF, Otsuka Pharmaceutical contributes to the health of people around the world through collaborative initiatives with its many stakeholders. These include: our participation in the Global Health Innovative Technology Fund (GHIT); a Japanese public–private partnership established to address infectious diseases worldwide; and cooperation with programs spearheaded by Médecins Sans Frontières/Doctors Without Borders (MSF). We have also received grants from the Bill & Melinda Gates Foundation to support clinical developments for a novel regimen of TB treatment. As for global access, we have built aliances with international companies which have strengths in public health or in areas where the Otsuka group does not have business bases, such as Viatris in India, South Africa and other countries with a high burden of TB and R-Pharm in Russia/CIS region.
Initiatives in which Otsuka Pharmaceutical participates
Rome 5
a Vatican initiative historically focused on children living with HIV & now including TB.
https://www.paediatrichivactionplan.org/
Ending Workplace TB
a collaboration of the World Economic Forum, the Global Fund and private sector companies to end TB in the workplace. Through internal and external communication drives, Otsuka Pharmaceutical has pledged to raise awareness of the disease with the public and its own employees in countries with a high burden of TB.
https://www.weforum.org/projects/ending-workplace-tuberculosis
Otsuka Pharmaceutical and Viatris are completing technology transfer aiming for production in India in order to ensure affordable access to delamanid in high-burden countries.
In March 2017, we launched a delamanid clinical access program (DCAP) in cooperation with the South African government. The aim of the national program was to give patients with MDR-TB swift access to delamanid before regulatory approval. Delamanid is now approved and widely available in South Africa.
In other initiatives, Otsuka Pharmaceutical is working to establish sustainable drug delivery systems at affordable prices, so that patients around the world who need delamanid can be treated regardless of socioeconomic status or income level. As of 2021, more than 110 countries are expanding use of delamanid based on this multifaceted approach.
Continuous Engagement for TB Drug Discovery
Next-generation tuberculosis drug candidate, OPC-167832
Otsuka Pharmaceutical is conducting research into anti-tuberculosis drugs that will follow delamanid. The latest development is a compound called OPC-167832, which as of 2021 is undergoing initial trials in South Africa to confirm its safety and efficacy.
OPC-167832 kills TB bacteria through a mechanism that inhibits the activity of enzymes that are essential for synthesizing mycobacterium tuberculosis cell walls. Because its mechanism of action differs completely from those of existing anti-tuberculosis drugs, including delamanid,it is expected to be effective as a treatment for various strains of TB. In developing the drug, we received grants from the Bill & Melinda Gates Foundation, which has cited elimination of TB worldwide as one of its top priorities, to advance clinical trials. We will continue engaging in TB R&D with the aim of establishing innovative treatment methods.
Initiatives in which Otsuka Pharmaceutical participates
The Project to Accelerate New Treatments for Tuberculosis (PAN-TB)
the first collaboration among philanthropic, non-profit and private sector organizations, led by the Bill & Melinda Gates Foundation. It aims to accelerate the development of an investigational drug regimen capable of treating all forms of TB (a pan-TB regimen).
Unite4TB
another public-private collaboration supported by the European Innovative Medicines Initiative (IMI) to accelerate development of novel TB combinations.
EU-Pearl
which aims to make drugs trials more efficient and patient-friendly.
https://www.imi.europa.eu/projects-results/project-factsheets/eu-pearl
Fighting Antimicrobial Resistance (AMR)
The Economist Intelligence Unit, in its 2019 report “It’s Time to End Drug-Resistant Tuberculosis, 11 asserts that, by the year 2050, drug-resistant TB could account for 2.5 million deaths annually*9.
Otsuka Pharmaceutical has been implementing the Responsible Access Program to prevent the outbreak of delamanid-resistant TB and to promote proper use of the drug.
Our stewardship achievements, including efforts to educate about proper use, increase global access to delamanid as well as continuous research and development into drug-resistant TB, are acknowledged in the AMR Benchmark, a survey of 17 global pharmaceutical companies.*10 The Benchmark is compiled by the Access to Medicine Foundation,and is funded by the UK and Dutch governments.
- *9The Review on Antimicrobial Resistance. Tackling drug-resistant infections globally: Final report and recommendations. London: Wellcome Trust and HM Government, 2016. Available from:
https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf - *10https://accesstomedicinefoundation.org/amr-benchmark








