Core Principles
The Otsuka group believes that we have a responsibility to deliver higher-value products and services to customers. To do this, we will engage in suitable promotion and advertising activities that comply with all laws and regulations, and communicate with customers in an effective way.
Promotion System for Pharmaceutical Business
Basic Approach and Policy
We have established standards of conduct that comply with the International Federation of Pharmaceutical Manufacturers & Association’s Code of Practice and the codes of industry associations in each country and region to ensure a high level of ethics and transparency in interactions with stakeholders, including medical practitioners, medical institutions, and patient groups, and to meet the trust placed in us by society. For example, in Japan, every company has developed its own code* that reflects and improves upon the Japan Pharmaceutical Manufacturers Association (JPMA) Code of Practice and Promotion Code for Prescription Drugs, mainly for sales divisions. In addition, all activities of the Otsuka group from R&D to providing medical information and other corporate activities are subject to laws and regulations, such as the Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices (Drugs and Medical Devices Law). We also comply with the Fair Competition Code concerning Restriction on Premium Offers in Ethical Pharmaceutical Drugs Marketing Industry (Fair Competition Code), voluntary codes such as the Guidelines for Provision of Sales Information on Prescription Drugs issued by the Ministry of Health, Labour and Welfare, administrative notifications, and other guidelines. Our compliance ensures responsible promotional activities based on high ethical standards suitable for a health-related company.
- *Otsuka Pharmaceutical Code of Practice
- *Otsuka Pharmaceutical Factory Code of Practice
- *Taiho Pharmaceutical Code of Practice
Promotion System
All promotional activities of the Otsuka group comply with laws, regulations, and guidelines.
For example, Otsuka Pharmaceutical, under the guidance of top management, has established a review and supervisory committee that includes a third party and an investigation committee within the supervising department that includes administrative and human resource sections. We have also introduced a system for regular reviews to ensure that promotional materials comply with all appliable laws, regulations and guidelines. In the unlikely event of a violation, an investigation is undertaken swiftly, and guidance is provided to prevent a reoccurrence.
Review System for Providing Sales Information (Example: Otsuka Pharmaceutical)
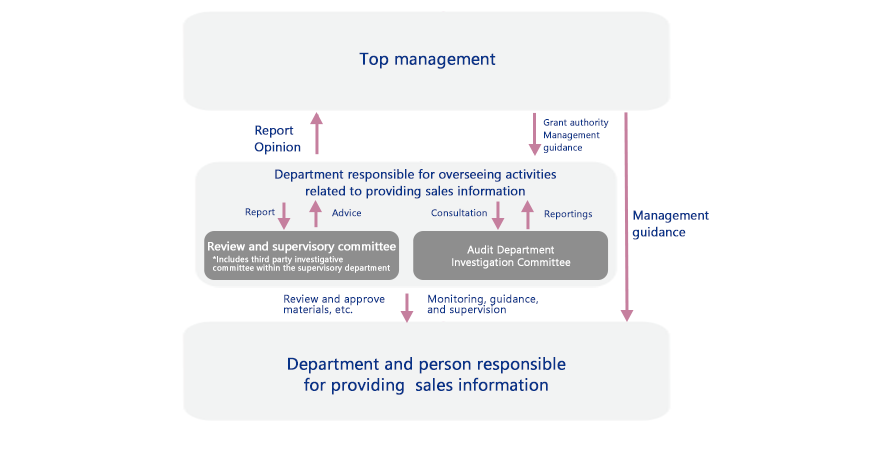
Review System for Responsible Promotion of Prescription Drugs
The Otsuka group has established a department to supervise how sales information is provided for prescription drugs to ensure that promotional activities are conducted properly.
For example, at Otsuka Pharmaceutical, the compliance promotion committee meets once a year and includes the participation of company presidents and other directors in the value chain. In addition to reporting results from a review of activities related to information distribution and information training, top management are also given training. The compliance promotion committee closely follows the advice of external members (lawyers) when providing sales information.
The Compliance Division takes charge of review and supervisory committee meetings twice a year that also include outside experts. The meetings cover the Company’s response to reviewing reports on information distribution as well as the current state of activities. All promotional materials for prescription drugs are reviewed monthly by a committee that screens the product information. The committee includes outside experts but no sales representatives to ensure objectivity. In addition, a limit is set for how long materials that have passed the review process can be used, with any extension requiring a separate review.
Further, the slides used by medical professionals for lectures are checked in advance by the Medical Affairs Department.
Training System for the Responsible Promotion of Prescription Drugs
The Otsuka group holds regular training for all employees involved with promotions, including departments that prepare materials, and sales representatives, with the aim of raising awareness of compliance. We also provide regular training for employees so they can acquire knowledge of the laws, guidelines, and ethics related to the prescription drugs and other products that we handle, as well as any other knowledge considered necessary. In the unlikely event of a violation, special training is given as soon as possible to prevent a reoccurrence.
We provide periodic training on the Fair Competition Code, Guidelines for Provision of Sales Information on Prescription Drugs and Guidelines for Transparency of Relationship between Pharmaceutical Companies and Medical Institutions, etc. to those from Otsuka group companies that handle the sales and promotion of pharmaceuticals.
Implementation of Training on Sales and Promotional Activities for Pharmaceuticals (Example: Otsuka Pharmaceutical)
| Participants | Main content | Frequency |
|---|---|---|
| Medical representatives, personnel affiliated with pharmaceutical sales division, etc. |
|
|
| Personnel in charge of preparing materials |
|
|
| Pharmaceutical sales division, medical affairs department, academic affairs department, etc. |
|
|
| Medical representatives |
|
|
|
|
Approach in the Nutraceutical and Consumer Products Businesses
Basic Approach and Policy
The Otsuka group, as a total healthcare company, conducts promotional and advertising activities in compliance with relevant laws and regulations of each country we operate.
In our nutraceutical business and consumer products business, we advocate the Otsuka Group Marketing Communication Policy. Each operating company carries out initiatives tailored to its own business characteristics under this policy.
Otsuka Group Marketing Communication Policy
- Providing information based on customer perspective
We strive to provide accurate and easy-to-understand information about our products and services. - Compliance
We establish a review system and comply with relevant laws, regulations, and self-imposed standards. - Respect for Diversity
We maintain high ethical standards and respect the diversity of our customers. - Contribution to Health
Through responsible, customer-oriented marketing and communication activities, we contribute to enriched lives and better health of people around the world.
Review System for Responsible Promotional Activities
The Otsuka Group has established a review system to ensure appropriate marketing and sales promotion activities in accordance with laws and regulations.
At Otsuka Pharmaceutical, the product information committee, chaired by an executive officer and consisting of managers from multiple departments such as administration, legal affairs, production and consumer-relations, meets every month to review sales promotion plans and advertising materials. The secretariat, meeting under the production information committee, which also includes staff from multiple departments, discusses individual sales promotion plans for each week.
At Otsuka Foods, the label review committee, also with people from different departments, meets once a week to review sales promotion plans and advertising materials. In addition, a debriefing session is held each month to share with management the content discussed by the committee. Directors from departments related to general affairs, legal affairs, and intellectual property as well as product managers and division heads take part in these debriefings.
Training System for Responsible Promotional Activities
To instill ethical compliance at group companies, the Otsuka group conducts universal training based on the content of the Global Code of Business Ethics, the Global Anti-Corruption Policy and the Global Conflict of Interest Policy. Officers, employees, contract employees, and dispatched employees at each operating company attend related training once a year. The levels of understanding of compliance and its penetration among employees are evaluated through a test during training, awareness surveys, and other measures. We also provide employee training on responsible sales and promotions that specifically focus on the business of each company.
At Otsuka Pharmaceutical, in addition to annual compliance training for all employees, we provide training with content specific to business divisions as well as training on laws, regulations and guidelines related to prescription drugs. By doing this, we are fostering an awareness of the need for strict promotional activities among all employees of a division from the perspective of a pharmaceutical company. Employees can also check regulations on promotional activities at any time on the company intranet.
Otsuka Foods raises awareness of compliance by sending emails on different topics twice a month and runs remote (online) compliance-related training for all employees five times a year to foster a comprehensive awareness of compliance.








