“What medicine do you really wish existed?” In 1983, Akihiko Otsuka, president of Otsuka Pharmaceutical at the time, asked this question of a doctor he happened to meet at an airport. “I want a diuretic that only excretes water,” was the doctor's immediate reply. Hyponatremia is a condition in which excess water retention linked to heart failure or hepatic cirrhosis causes dilution of blood sodium. This condition can trigger seizures or convulsions and may be life-threatening. In those days, no effective therapy existed for hyponatremia and patients were subjected to stressful regimens that limited fluid intake.
An orally administered drug that promoted excretion of only water while limiting excretion of electrolytes: development efforts focusing on meeting these medical needs were far from simple. In the search for a compound that inhibited reabsorption of fluids, identification of a promising compound that became the starting point for focused development took three years. The repeated process of “producing and testing” thousands of compounds continued until 1991, when the structure of the compound which would become the new drug was identified. Eight years had passed from the project launch. According to one researcher, “our company showed great patience, offering fertile ground for continued efforts to develop something beneficial for patients and society.” Developed through tenacious research, a “diuretic that promotes excretion of only water” was launched for treatment of hyponatremia in the U.S. in 2009. In Japan, it was approved for treatment of edema from heart failure in 2010 and for hepatic cirrhosis patients in 2013. A quarter of a century after the initiation of the project, the determined efforts of researchers bore fruit.
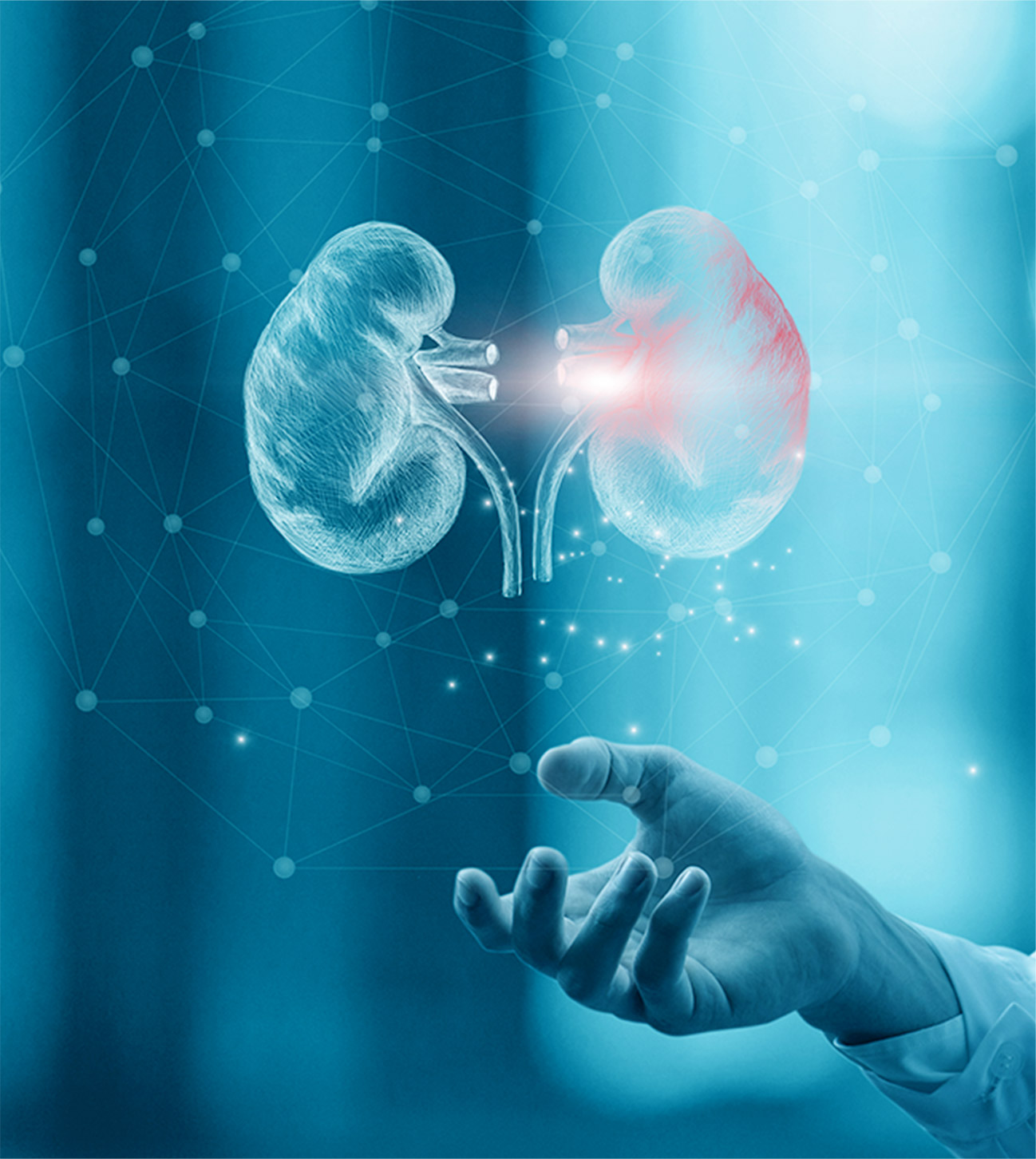
In the course of research into this new compound, potential for treatment of an entirely different disease became apparent. The compound showed potential as a new treatment for autosomal dominant polycystic kidney disease (ADPKD), a rare genetic ailment. At that time, ADPKD was a relatively unknown disease, with no established treatment options. Few physicians had the ability to accurately diagnose this intractable disease. Clinical studies for treatment of ADPKD were launched in 2004. Teams in Japan, the U.S. and Europe conducted the first collaborative clinical study with over 1,400 patients in 15 countries.
These large-scale clinical studies began in 2007. Results presented at the American Society of Nephrology in 2012 were published in an international academic journal. In 2014 the world's first treatment for ADPKD was approved in Japan, but various hurdles remained before approval could be obtained in the U.S. Convinced that “patients in the U.S. should benefit” from the compound, researchers completed painstaking preparations for a second round of large-scale clinical trials, leading to approval in the U.S. in 2018.

“First in class.” In the pharmaceutical industry, this term is used to describe highly effective innovative new drugs with novel mechanisms that have the potential to change the therapeutic landscape. Development of such first-in-class drugs requires immeasurable time and unimaginable dedication.
A new diuretic contributes to treatment of edema in patients with heart failure or hepatic cirrhosis and also serves as a therapeutic drug for ADPKD, a rare disease that previously had no treatment. Developed through the tenacious efforts of Otsuka researchers, this innovative global drug has been approved in more than 40 countries and regions.