Tuberculosis (TB) is an old disease with a new face. Once feared as an “incurable malady,” today TB can be treated, but it continues to be a menace that infects as many as 10 million persons worldwide every year, resulting in more than 1 million deaths. TB is caused by Mycobacterium tuberculosis, and treatment with multiple drugs over a prolonged period is required to eliminate infection. Easily transmitted through the air, the bacterium is difficult to control. Mutations may result in the proliferation of strains that are resistant to existing treatments. And development of TB treatments is extremely difficult.

Otsuka Pharmaceutical began drug discovery in 1971. One of the central themes was treatment of infectious diseases, including TB. During the early 1970s, many pharmaceutical companies had halted efforts to develop TB treatments. Encouraged by a new TB drug discovery, companies shifted the focus of their research to other areas that promised higher profits. However, despite the development of the new drug, TB remained a major public health problem. In the absence of ongoing research, TB could once again become a widespread menace, threatening human lives. “We must not abandon TB drug discovery research.” Otsuka researchers were motivated by this strong conviction.
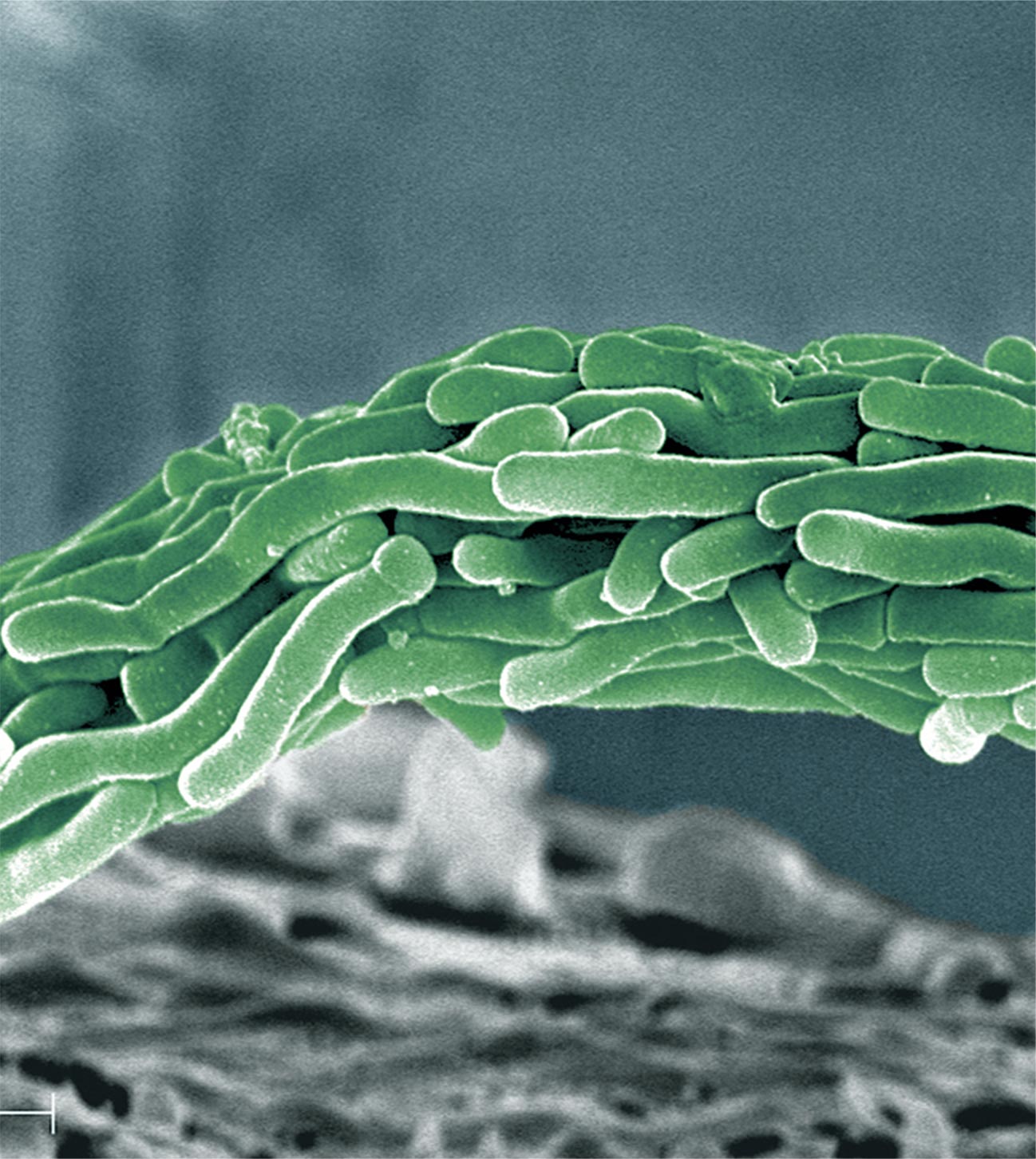
The research was demanding. Results were elusive and suspension of the project was considered. But fueled by the possibility of making a unique contribution, Otsuka researchers continued to focus on TB. Convinced that “someone must continue this research,” decades of dedication led to discovery of a substance that was highly effective against TB, including strains that had developed resistance to existing treatments.
In 2014, this treatment was approved in the EU and Japan, one of the first anti-TB drugs to receive approval in about 40 years. Japan's first treatment for multidrug-resistant TB, this new drug was added to the WHO Model List of Essential Medicines in 2015. In partnership with the Stop TB Partnership's Global Drug Facility and overseas pharmaceutical firms, distribution was expanded to countries where TB is widespread, leading to use in more than 120 countries and regions worldwide. But release of a new TB treatment is only the beginning. Irregular administration or discontinued treatment can hasten development of drug-resistant strains, which limits treatment options and increases the difficulty of treatment. Otsuka Pharmaceutical is working together with partners and government organizations to promote public awareness of optimum use of TB treatments.
The fight against TB, the old disease with a new face is not over. Treatment for children suffering from multidrug-resistant TB is difficult. Otsuka has developed a formulation for children and is working on expanding indications for use. Through participation in international partnerships, Otsuka has also begun research and development of an anti-TB agent with a new mode of action. Aiming to protect humanity from the ravages of TB, Otsuka continues research and development that “someone must continue.”










